Answer : The formal charge of each oxygen atom in ozone are 0, +1 and -1 respectively.This Site Might Help You. RE: Based on the formal charges added, which HCNO structure is favored? There are three structures below: One is the original organic formula: H---C----N---(x3 bond)OThe formal charge of the ozone molecule is zero. Its Lewis structures do present charge separation.
Based on the formal charges added, which HCNO structure is
In order to calculate the formal charges for O3 we'll use the equationFormal charge = [# of valence electrons] - [nonbonding val electrons] - [bonding electr...Organic Chemistry Chapt 1.docx - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free.A formal charge is equal to the number of valence electrons of an atom MINUS the number of electrons assigned to an atom. Consider the resonance structures for O3. Oxygen has 6 valence electrons. Look at the top left oxygen atom.

How do you calculate the formal charge of O3? | Socratic
A step-by-step explanation of how to draw the O3 Lewis Dot Structure (Ozone).For the O3 structure use the periodic table to find the total number of valenceO3 Lewis Structure Formal charges Resonance structures VSEPR structure Orbital boundary-surface diagram (VBT) sigma and pi structures electronic box diagram for VBT central atom showing formation of hyb. 1 answer I have posted this question before but want to see if answers from different people match up. It's for a study guide please helpNow, a lot of people ask why it is necessary to know the Lewis structure of any given molecule or compound. The answer to this question is simple; this structure helps in understanding the basic structure, electrons that take part in bond formation along with the charges on a given atom.
A formal charge is equal to the number of valence electrons of an atom MINUS the selection of electrons assigned to an atom.
Consider the resonance structures for #"O"_3#.
Oxygen has #6# valence electrons. Look on the best left oxygen atom. It has two lone pairs (#4# electrons) and a double bond (#2# electrons).
Even though a double bond incorporates #4# electrons overall and is counted as such when seeing that oxygen's octet is crammed, #2# electrons belong to every oxygen and they're shared among the two.
Let's read about the highest resonance structure:
Left: #6# valence #-6# assigned #=colour(blue)(0# formal chargeCenter #6# valence #-5# assigned #=colour(blue)(1# formal chargeRight: #6# valence #-7# assigned #=color(blue)(-1)# formal price
Notice that even though the atoms have varying formal charges, the whole charge of #"O"_3# is the sum of the formal charges within the molecule: #0+1+(-1)=0#.
Ions' formal fee sums are #!=0#.
01g

IB Chemistry Resonance, Delocalization For Ozone And Benzene - [PDF Document]
![IB Chemistry Resonance, Delocalization For Ozone And Benzene - [PDF Document] IB Chemistry Resonance, Delocalization For Ozone And Benzene - [PDF Document]](https://i0.wp.com/demo.documents.pub/img/378x509/reader024/reader/2021011017/547be3d75906b59f798b4652/r-1.jpg)
Solved: What Formal Charges Are There On The Following Lew... | Chegg.com

3.4 Covalent Bonds And Lewis Structures - Columbia Bonds And Lewis Structures Formal Charges •Formal Charge Is The Charge Calculated For An Atom In A Lewis Structure On The Basis - [PDF Document]
![3.4 Covalent Bonds And Lewis Structures - Columbia Bonds And Lewis Structures Formal Charges •Formal Charge Is The Charge Calculated For An Atom In A Lewis Structure On The Basis - [PDF Document] 3.4 Covalent Bonds And Lewis Structures - Columbia Bonds And Lewis Structures Formal Charges •Formal Charge Is The Charge Calculated For An Atom In A Lewis Structure On The Basis - [PDF Document]](https://i0.wp.com/demo.fdocuments.in/img/378x509/reader018/reader/2020011121/5a840ce57f8b9ada388f00c8/r-2.jpg)
CHEM2131 Day 1 Worksheet Key - StuDocu
Chemistry Net: Simple Procedure For Writing Lewis Structures- CO2, NCO- – Examples #2
Formal Charge
Practice Test I Bonding And Geometry

NCERT Chemistry 1 Class 11 By Junaid Fardeen - Issuu

Chapter 8: Concepts Of Chemical Bonding
Co Re)
Solved: 2. Draw The Lewis Structure For The Ozone Molecule... | Chegg.com
Lewis Diagrams -- Formal Charges What Is The Formal Charge On The C Atom In The... - HomeworkLib

Solved: 2. Draw All Possible Resonance Structures For O3 A... | Chegg.com
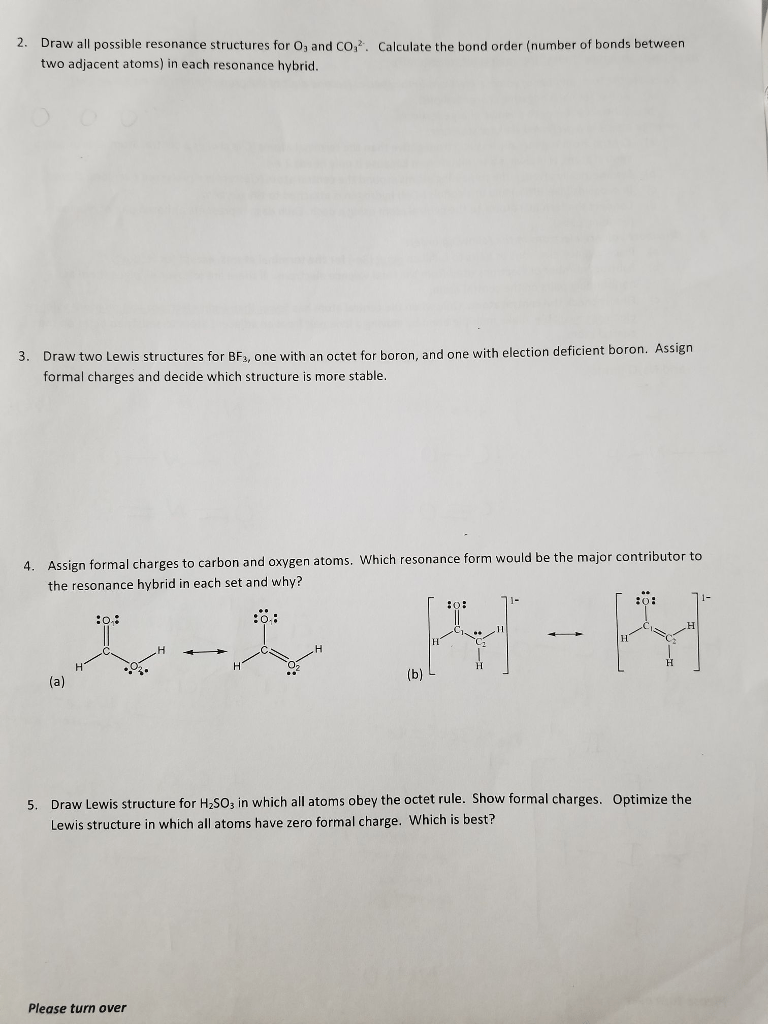
1 TN, Pr
Untitled
How Many Resonance Structures Does N3- Have? | Socratic

Key For Take-Home Assignment 04
Answered: Confirm That There Is No Legitimate… | Bartleby

Untitled
O3 Lewis Dot Structure (Page 7) - Line.17QQ.com

0 comments:
Post a Comment